Introduction to Electroplating: The Historical Perspective
Although the electroplating process looks flashy and high-tech, electroplating is a traditional, industrial technique that originated in 1805. The development of the concept was influenced by early scientists exploring the aspects of electronics and their practical applications. The initial practice was mainly a test of whether it was possible to get the reaction to occur and included such minerals as rust. The experimental stage was mostly trial and error, but there were many by-products such as oxides and chlorides of the parent chlorides, making the discovery a positive feature; otherwise, it would have been forgotten.
Meeting the need for a new jewelry material, the process became industrialized starting in the early half of the 19th century. As none of the materials for its production are limited or expensive, the technique offers a sustainable alternative to using resources with a shorter lifespan. The technology, developing at a fast pace, gives more opportunities for the use of the technique in various fields besides the traditional ones where it is necessary but not so important.
Other metalworking methods aren’t the only costly, time-consuming ones: electroplating falls into this category too. If you look at the story of electroplating, it’s an impressive testament to human curiosity for experimenting and the eager desire for advancement in tech. The most fascinating aspect of electrodeposition in electro-metallurgy is its usage across a variety of sectors. The first uses of electroplating were mostly in the textile industry on intricate items. However, with the birth of the synthetic organic chemical sector, unique chemicals were used to separate fibers during bleaching. X-ray diffraction creates a denser soil with more pores and is more permeable.
A bonus of mapping metalloproteins is the reduction of diseases caused by metal deficiency, offering precise intel about the role of the protein in the metal pathway. Fractionation, a method of extracting fuel from hydrocarbons, has many benefits including the separation of metal processing and environmental issues. We can ensure a clean, pollution-free future by buying shared spaces and reducing road traffic. From ancient days to now, electroplating has witnessed massive tech applications and is continuously moving towards automation, opening it to new fields.
The ever-evolving story of electroplating is tied with our constant pursuit of the future, seeking educational technology and continuous tech enhancement. The advancement in tech, variety in studies, and spread of electroplating have made this process vital to both the present and future world. The introduction of advanced materials in this era confirms that despite the first point being valid, electroplating remains a dynamic, business-centric method for creating decorative items. Although in the beginning, it wasn’t quite similar to today’s construction business, the trend indicates the future potential of structural materials based on the electroplating technique. Environmental challenges like global warming, ozone layer depletion, deforestation, and more are critical: we’re on the brink of an environmental crisis.
In the exploration of electroplating, we will dig deep into the roots of its science, discovering the ionic processes that take place at the atomic level. We shall discuss its application in a number of industries and evaluate the carbon footprint and other environmental safety hazards associated with the technology at scale alongside safety gear that is necessary for its operation. We are going to point out some of the fascinating capabilities that electroplating can achieve with the development of novel techniques.
Let’s take a walk into this tour and know more about the nuts and bolts of electroplating, the story of what has been, is, and will be.
Electroplating is a process that uses electricity to apply a thin layer of metal to an object. It might be metals like gold, silver, or nickel. This process as a result makes the product look magnificent, strengthens it, and helps reduce friction while also enhancing conductivity. In the manufacturing of automobiles, jewelry, electronics, as well as in the medical and chemical industries, among others, the use of electroplating has been one of the most essential processes in the making of the products of contemporary society.
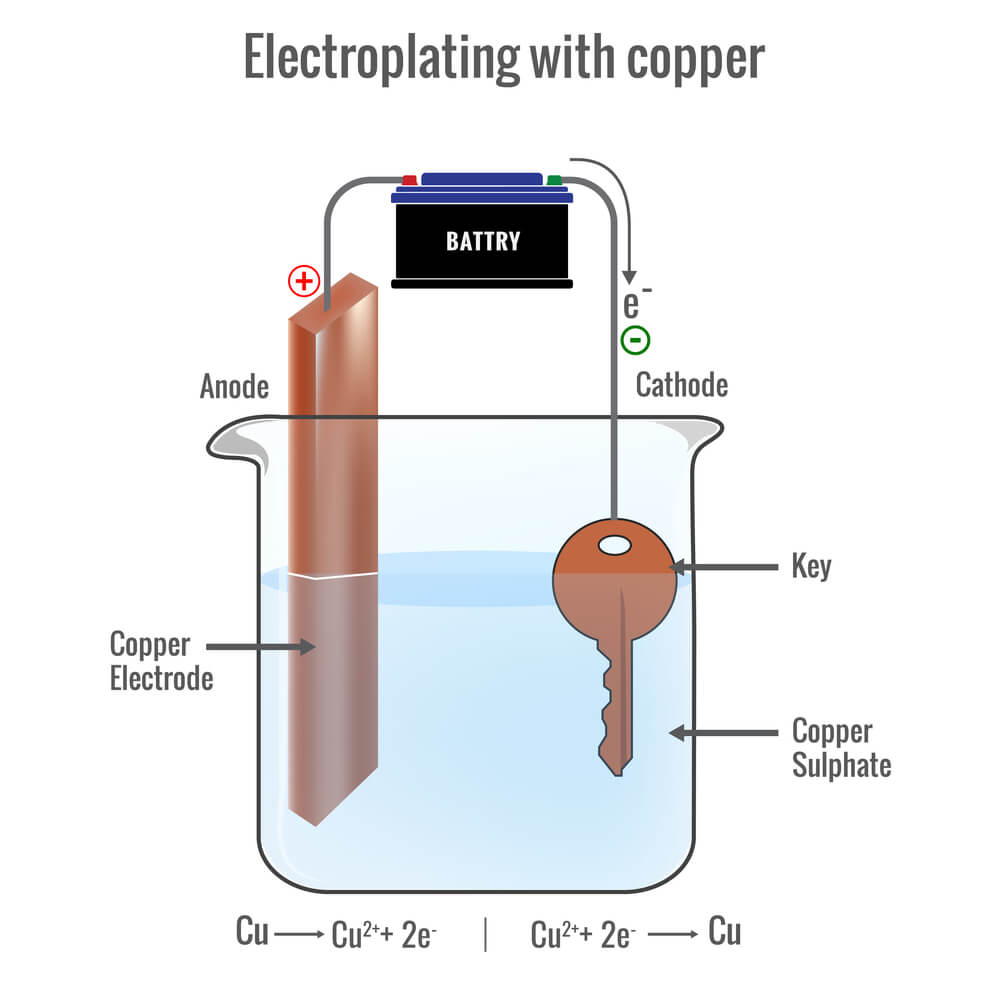
Understanding the Electroplating Process: The Science behind the Technique
The electroplating process seems to be a really complex one, which actually relies on the fundamentals of science. At its basic state, it is a process where the metal is coated with a mixture of cations through the use of electric currents. These cations are melted, and the metal coating sticks to the electronic component through a chemical reaction that is taking place. For instance, if the silverware is a cathode, then the activating electric charge will make the base metal a still a workpiece while the material to be plated a conductor up to ionic form.
Electroplating is the process of dipping the workpiece and the anode in the electrolyte solution. By electrolysis, the solution with the metal salts required to cause the galvanizing process initiates the flow of electrical current between the anode and the cathode. The Thomas-Edition metal ions would deposit the workpiece under current.
Moving metal changes due to the speed at which the metal plate goes to and also the covering of the metal with the time during which the workpiece is within the bath. The tight control of the parameters results in the coating of the proper thickness if the current is adjusted for the same period of time as the others.
This will be the reason for the success of the electroplating process. Electroplating will not go ahead with the workpiece being cleaned completely, thereby aligning the need for removing oxides, grease, or other types of contamination. Mechanical cleaning, acid cleaning, ultrasonic cleaning, and electrocleaning are involved.
All in all, while the electroplating—a relatively straightforward process—in theory, it’s a piece of cake that is out of the question. The aesthetics, thickness, the hardness of the product, and the distance of the layer on the substrate will be mainly defined by the solution’s properties like anodic material, temperature, current density, and the electrolyte’s half-reaction.
Furthermore, in a study, the following confirms, the various types of electroplating that take place in a given location with a specific set of materials and conditions whose characteristics are enforced with it unrecognized much by human beings are of interest.
Have you ever wondered how electroplating was first discovered? Well, it was actually an accidental discovery made by an Italian physicist named Luigi Galvani. He was carrying out an experiment in which he connected two metals of different kinds and observed that one metal was coated with the other. This discovery not only operates in the electroplating world but it has been a significant contributor to the modern development of electroplating using technology.
Important Components in the Process of Electroplating
Important parts in the process of electroplating
Aside from the intricate procedure that it has, electroplating also consists, in part, of a serial network of processes that ultimately are the conditions of acceptance or rejection of correctness of the finished items. The fact that it works shows the fact that it utilizes a substrate, anode, electrolyte, power source, and an environment in which it operates.
Substrate
The substrate is a certain surface that will be placed under the action of an electric current during the electroplating process. There can be different examples of substrates such as a piece of jewelry or a part for an electronic gadget. Various substrates have specific preparatory procedures which may include degreasing, pickling, and buffering with the purpose to support the bonding of metal salts preferable in specific areas.
Anode
Surely taking the fundamental position, an anode is thus the metal from the one you desire to be deposited on the workpiece. It is mainly the trap of ions for the metal into the electrolyte because the releasing of the ions takes place there on the anode.
Electrolyte
The electrolyte is the mixture of metals that contain the metal ions from the anode and bring them to the substrate. More often than not, it is mixed with other chemicals that play the role of the catalyst for the metal layer to be crystal-clear, strongly adhered, and of the desired texture as well.
Different factors such as the arrangement, temperature, and the pH of the electrolyte alone are the influential elements that are directly changing the quality of the electroplated layer.
Power
A direct current power source is the one needed for the process. The depletion rate of the metal ions, the thickness, and the smoothness of the electroplated layer can be
varied through the adaptation of the voltage and current parameters both down and up.
Operating Environment
The final thing to be noted is the operational environment where the control is through the factors such as temperature, mixing, and time respectively that significantly influence the process. The uniform fluctuation of temperature is seen as the cause for the process proceeding well but the final product properties are the deciding factors of the defects.
Electroplating problems may be minimized and solutions can be found to what is thought to be issues with defective end products and low productivity levels. In the following parts, we shall try to provide a detailed interaction regarding the types of electroplating and the relationship of the key elements to them.
RehumanizeTypes of Electroplating: From Gold and Silver to Nickel and Zinc
Electroplating has been accomplished with various sorts of metals, each steel amongst such particularly a characteristic tempo progressing finished goods. The choices to use which metal, on the other hand, strongly depend on how the object of interest is associated during the procedure. Some of the most frequently used types of electroplating are listed hereunder:
| Metal | Applications | Characteristics |
|---|---|---|
| Gold | Electronics, Jewelry | High electrical conductivity, corrosion resistance, aesthetic appeal |
| Silver | Electronics, Cutlery, Jewelry | Excellent electrical conductivity, aesthetic appeal, antimicrobial properties |
| Copper | Electronics, Industrial | High electrical and thermal conductivity, facile plating |
| Nickel | Automotive, Industrial | Corrosion resistance, hardness, resistance to wear |
| Tin | Food Packaging, Electronics | Non-toxic, solderability, resistance to corrosion |
| Zinc | Fasteners, Automotive | Resistance to corrosion, sacrificial protection |
| Chrome | Automotive, Decorative | Hardness, appearance, resistance to corrosion |
 Gold Electroplating: When a development stage called rapid prototyping requires a product to have several of the benefits that would come from expensive solid gold, this is done through gold electroplating, which combines the advantages without the cost. Gold electroplating is used in the electronics industry and jewelry making. It is different from other high-reliability applications because it is utilized for its high electrical conductivity, excellent layer adhesion, and corrosion resistance.
Gold Electroplating: When a development stage called rapid prototyping requires a product to have several of the benefits that would come from expensive solid gold, this is done through gold electroplating, which combines the advantages without the cost. Gold electroplating is used in the electronics industry and jewelry making. It is different from other high-reliability applications because it is utilized for its high electrical conductivity, excellent layer adhesion, and corrosion resistance.
Plating methods used on gold have made it difficult to know which methods pose serious threats to the environment, people, and animals. The elite class houses may have the information, but the paper will post the reality of the treatment.
Silver Electroplating: Being among the most conductive materials to electricity, silver electroplating is often employed in electronics, specifically in interconnects, switches, and contacts, which are all components of electronic devices. Silver plating, apart from its traditional use in electrical appliances, also represents another method of decoration, for example, in silverware and jewelry. Silver metal has major potential, and its significance mostly depends on the use of these products.
Copper Electroplating: Copper electroplating forms the core of most applications that call for high thermal or electrical conductivity. For example, it is the primary application in the electronics industry where copper plating helps to create conductive paths on printed circuit boards. It is also one of the metals which are easily plated with virtually every product, hence its popularity among others.
Nickel Electroplating: Nickel electroplating is a widely used process, both past and present. It is a good composite for processing machinery and machine parts exposed to severe environmental conditions such as oxidation and wear, as nickel offers corrosion and wear resistance. Plating with nickel results in the longer service life of steel, iron, and brass automobile parts. Additionally, washers covered with nickel plating are attired in nickel because of its shiny surface, which has the reflective property of light.
Tin Electroplating: Tin is a material that is not only non-toxic but also allows soldering to the two-day-old circuit board, making it usable in the food processing industry and electronics. Tin is utilized in food packaging along with electroplating. Through electroplating, corrosion is prevented on the surface, ensuring food is released in a good state. Soldering connects components in the circuit, where a piece of copper absorbs heat and is soldered into place to function properly.
Zinc Electroplating: Zinc electroplating prevents rust on steel or iron parts of products. The zinc plating first reacts with surrounding oxygen, sacrificing the metal to oxidation and corrosion. Initially used as a finish for brass, its powers in the automobile industry and the production of fasteners like bolts and screws have led to its wide application.
Chrome Electroplating: Chrome electroplating stands out for its shiny and attractive look and extreme resistance to wear. It is mainly found in automotive parts, including light and bumper finishes, reflector housings, and worn batteries. The general weathering and blistering occurring in airframes prompted the removal of perfectly good skins and their replacement, much higher than electron screening suggested.
Work on tweaking the electroplating technique in fashioning the final product should be your choice, but first, you need a solid grounding in analyzing the factors behind such interventions. Whether it’s the look, smoothness, wear resistance, or viable techniques crucial to electroplating, they cater to a wide range of industries and are inevitable for almost every one of them.
Applications of Electroplating: How It Shapes Our World
Electroplating is practically everywhere you look, with a myriad of uses that have transcended many different industries. It finishes surfaces, ensures a certain level of conductivity of modern parts, and is used as a coating for tribocorrosion tests.

Jewelry and Decorative Items: One of the most ancient and popular technological operations involving electroplating is in jewelry and decorative items. Products coated with gold and silver, which are base metals, will have the same look and feel as precious metals but won’t be as expensive. Electroplating adds to the life of these products, ensuring durability.
Automotive: Electroplating features a wide range of applications in the automotive industry. Different components such as bolts and screws are manufactured with a surface of metals like nickel, zinc, or chrome, making them impervious to corrosion and boosting performance.
Electronics: The increased use of electronic devices in our lives has led to increased electroplating in this area. It is a crucial process that provides the necessary conductive tracks on printed circuit boards (PCBs). Metals like gold, silver, and copper are used to increase conductivity and consistency.
Aerospace: Electroplating technology occupies a significant position in the aerospace sector with various applications. It protects aerospace components from extreme temperatures, corrosion, and wear. Plating enhances the safety and functionality of space equipment.
Healthcare: Electroplating has found widespread usage in the healthcare industry, almost becoming a basic medical method. Medical devices and instruments require strong coatings, reduced wear, and resistance to bacteria. Silver’s antifungal properties ensure surgical instruments are safe and effective.
From these illustrations, it is clear that electroplating is crucial in various applications and industries. It is a fundamental requirement for new knowledge and the dynamic pursuit of better products through diverse properties. As advances continue, the potential for innovation in electroplating technologies is vast.
Environmental Aspects of Electroplating: Challenges and Innovations
Like other industrial practices, electroplating has some negative environmental impacts. Traditional electroplating uses toxic heavy metals and hazardous chemicals, creating substantial amounts of waste. However, the shift towards eco-friendly methods is gaining momentum.
Waste Management: One major environmental problem in electroplating is the disposal of waste products, such as used or spent plating baths and rinse water. These waste products often contain heavy metals and other dangerous materials that can deteriorate the environment if not properly managed. Research on hazardous waste treatment helps reduce surface pollution by stirring concrete deeper into the ground.

Use of Hazardous Chemicals: Many electroplating processes utilize chemicals considered a big environmental threat, such as cyanides. Industry partners have been working tirelessly to minimize harm, with some designers creating cleaner, safer, and highly efficient plating baths.
Energy Consumption: Electroplating can be a high-energy process, particularly for certain types of plating. Instead of manual handling, developers prefer semi-automatic handling to reduce energy consumption.
Worker Safety: The use of hazardous substances in electroplating raises concerns about worker safety. The industry has implemented rigorous safety standards and continuously works to create safer work environments.
The electroplating industry faces environmental challenges but also holds opportunities for innovation. Recent trends focus on producing electroplating technologies that use less energy, produce minimal waste, and eliminate toxic chemicals. Automation and digital technologies can further automate the process, reducing environmental impact and ensuring high-quality, consistent finished products.
The industry’s growing response to green electroplating is a step forward. Replacing old soaking techniques with new ones is an example of reducing energy waste and improving efficiency.
RehumanizeThe Future of Electroplating: Trends and Emerging Technologies
In the case of electroplating, as the technology, environment and market needs change, it is bound to change and be transformed along several new pathsis bound to change and take a total different direction given changes in technology, environment and demands within the market. Here are some trends that will shape the future of electroplating:
Sustainable Practices:As already communicated earlier, the electroplating industry is more conscious of the need for sustainability. This includes not just waste reduction but also using safer materials and less energy, worker safety improvement. For example, research shows that many companies who engage in better environmental practices often find that sustainability even becomes a marketing tool for them to gain a competitive advantage in the green marketplace thus eco-friendly practices are becoming the norm for companies operating in the sector.
Rehumanize
Electrolyte Formulations-Innovations:Yet another big trend under the banner of green electronics is the whole new concept of innovating technology involving electrolyte formulations. These breakthroughs may be routes to better coatings, costs reduction, and wiser natural resources usage which in turn leads to better environmental performance. For instance, scientists are contemplating using ionic liquids which are special kind of liquid salt as a plating bath that replaces many or all of the existing ones. The result is the shallow penetration of the washing solution down into the pores of the fabricated piece and the elimination of toxic compounds from the environment
Nanotechnology: Nanotechnology in the sectors of electroplating is a moving front with great potentials. Electroplated products that are nanocoatings can give quality basic of a very hard nature among other properties of the traditional product. It might be in a new varieties of coatings with distinct properties, among other applications currently undiscovered.
Automation and Digitalization: Both automation and also the use of digital technology are more and more thought in the electroplating field. They accelerates process automation which makes it easier to manage processes, speeds up production, decreases mistakes, and cuts expenses. One of the ways in which automation enables advancements is through the use of continuous recording systems which have side by side the capability of monitor and adjust the plating parameters on a real-time basis so that it takes every time the same consistent high-quality result.
Adaptive Surfaces: The third one, which is fascinating research is adaptive or smart coatings. These coatings as if by magic change their properties when the balance “temperature. humidity. pressure” is disturbed. Such platforms can be applicable to a whole host of functions such as self-cleaning surfaces to self-healing materials that would repair themselves when they are damaged.
The upcoming directions of electroplating tell us that electroplating will be, in fact, not only more efficient or eco-friendly, but also it will be able to produce innovative products which are likely to perform and function much better than the prevailing products. There is no doubt that the electroplating sector will continue reaching unexplored horizons especially with the ever-increasing experimentation and innovation, which are expected to keep the project doors wide open.
Conclusion: Ubiquitous and Unseen Impacts of Electroplating
Engaged in the topic of electroplating fully, we have equipped ourselves with the knowledge of how working on such a background silently interacts with the basic elements of the world that surround us. It is in the jeweler’s equipment we wear, the materials used to make the vehicles we drive, the equipment we utilize in digital communications and even in the warfare systems of military installations. It is this one unit that is nonnegotiable across sectors. It is in the production itself, where the equipment effectively picks up the whole process and controls the production of household goods and circuit boards. The equipment helps extend the life of products by maintenance, optimization of power, and reduction of environmental impact.
Even though importance is not detruded, problems often surface in electroplating. Certainly, it should be dealt with in terms of both the environment and the workers’ safety. Good news! The market direction to greening has by this time deeply permeated the talks with the end-users and it is reinforced broadly in the industrial sector. The unending quest for green electroplating is not merely a responsible move but a catalyst in both technology and economics as well.
RehumanizeElectroplating is an area with quite a huge number of paths for different advance and present in the future the idea of new micro and nanotechnologies in digital and ICT sectors, fusions surfaces, smart material, etc. However, as we see and witness new technologies in the future, this treatment will be altered but will continue being essential for the development of our society and will give rise to further changes and new milestones in these sectors.
Frequently Asked Questions
Which technology is included in the process of electroplating?
Electroplating technology can also be termed as the use of electric current to deposit dissolved metal cations to form a coherent, thin metal coating on an electrode. This process is mostly employed for decorative finishing, corrosion resistance, and increasing hardness.
What does electroplating do to metal?
Electroplating can give different metal characteristics such as corrosion resistance, a brilliant finish, and hardness modification. It essentially enhances the metal properties, making it more durable and attractive.
What are 3 disadvantages of electroplating?
1) Electroplating often requires dangerous chemicals that can be harmful to people and the environment.
2) It can be a time-consuming process that requires a complex labor setup.
3) If not executed properly, it may not provide the intended coverage.
Does electroplating enhance the metal hardness and strength of a metallic item at the expense of its rust resistance?
Electroplating increases the hardness and strength of a metallic object, enhancing wear resistance. However, if the plating is damaged and the base metal is exposed, it can become susceptible to rust.
What is a negative outcome of electroplating?
The most harmful side effect of electroplating is the potential pollution of the environment due to toxic chemicals. Additionally, inadequate safety measures can pose serious health risks to workers.
Is electroplating rust-proof?
Electroplating can add resistance to corrosion, especially when metals like zinc or chromium are used. However, the metal can still be susceptible to rust under certain conditions.
Is it rust-proofing the metal on electroplating parts?
The electroplating process does not directly contribute to rusting; instead, it can help prevent it. However, if the plating is damaged and the base metal is exposed, rusting can occur.
Which metal cannot be electroplated?
Most common metals can be electroplated without any challenge, but aluminum can be problematic due to the oxide layer it forms when exposed to air, which can prevent successful electroplating.
How long does electroplating last?
The lifespan of electroplating depends on factors such as the type of metal used, the thickness of the plating, the environment, and how well the product is maintained.
Is electroplating expensive?
The cost of electroplating depends on the type of metal, the parts to be plated, their size, and complexity. Gold or platinum plating can be particularly expensive.
What is the strongest metal for electroplating?
Hard chrome is commonly used when maximum strength and durability are needed. The strength of the electroplated metal depends on the application and specific conditions.
Can you electroplate two different metals?
Yes, two different metals can be electroplated together, but it is complex. For example, aluminum requires special chemical treatment to ensure successful plating.
Can aluminum be electroplated?
Yes, aluminum can be electroplated, but it requires special treatment to remove the oxide layer before plating.
Can you electroplate stainless steel?
Yes, metals like gold, silver, or nickel can be deposited on stainless steel through electroplating to prevent corrosion and enhance magnetic properties.
Can magnets be electroplated?
Yes, magnets can be electroplated to provide an additional layer against corrosion and modify their physical and chemical properties.
Can you electroplate without electricity?
Electroplating requires an electric current to deposit metal ions onto an electrode. It functions through a direct current electrical source, causing electrochemical reactions at the anode and cathode.

